Abstract
Background: Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous hematologic malignancy with highly variable genetic abnormalities and clinical outcomes, highlighting the importance of individualized treatment. Previous studies showed that CD79B mutation was associated with higher risk of relapse and shorter survival after standard treatment (2020 ASH Abstract 2923), and both CD79B- or CD79A-mutant lymphoma cells were sensitive to BTKi in vitro (Nat Med. 2015;21:922-926.). Thus, we inferred that BTKi combined with immunochemotherapy might be a favorable option for pts with CD79A/CD79B-mutant DLBCL. Here, we present preliminary data from the phase 2 trial of Zanubrutinib combined with immunochemotherapy in pts with CD79A/CD79B-mutant DLBCL (NCT04668365).
Aims: To investigate the efficacy of Zanubrutinib combined with immunochemotherapy in pts with CD79A/CD79B-mutant DLBCL. The primary objective is complete response rate (CRR).
Methods: Pts aged 18-80 years with CD79A/CD79B-mutant DLBCL and adequate organ function are being enrolled. This study consisted of treatment naïve (TN) cohort and relapsed/refractory (R/R) cohort. Zanubrutinib (160 mg po bid) plus R-CHOP (ZR-CHOP) was administered in TN cohort, and Zanubrutinib (160 mg po bid) combined with investigator-determined conventional salvage chemotherapy (CSC, including ICE, DHAP, GDP, or GemOx, +/- rituximab) was administered in R/R cohort. A totally of 7 cycles of treatment was planned for TN patients. After 5 cycles of induction treatment, responders in R/R cohort undergo autologous stem cell transplantation (ASCT) or Zanubrutinib maintenance for 12 months, based on the patient's fitness and preference.
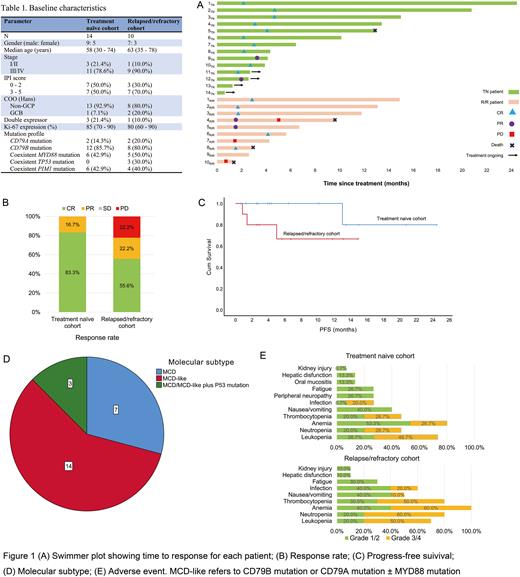
Results: From July 2020 to 22 July 2022, 137 pts have been screened, including 103 TN pts and 34 R/R pts. CD79A/CD79B mutation was identified in 26 (25.2%) TN pts and in 14 (41.2%) R/R pts, of which 14 TN pts and 10 R/R pts agreed to participate in this trial. Their baseline characteristics are displayed in Table 1.
By 30 July 2022, 12 TN pts and 9 R/R pts had been evaluated for response. There were 10 CRs (83.3%) and 2 PRs (16.7%) in the TN cohort; 5 CRs (55.6%), 2 PRs (22.2%), and 2 PDs (22.2%) in the R/R cohort. The median follow-up time was 6.4 (2.6 - 24.5) months for the TN cohort and 11.8 (1.4 - 14.9) months for the R/R cohort. One pt (5TN) died from non-tumor-related disease and no PD was observed in the TN cohort. In the R/R cohort, 3 pts experienced PD (all harbored TP53 mutations) and 2 of them (4R/R and 10R/R) died, another pt (8R/R) died from non-tumor-related disease.
The most common adverse events in both cohorts were hematologic toxicities. Grade 3/4 AEs occurred in ≥20% pts were neutropenia, leukopenia, anemia, thrombocytopenia, and infection. Bleeding and cardiac events were not observed.
Conclusion:CD79A/CD79B mutation was frequent in DLBCL patients, especially in R/R cases. Zanubrutinib combined with immunochemotherapy showed encouraging activity and acceptable tolerance in pts with CD79A/CD79B-mutant DLBCL. TP53 mutation seemed to be a detrimental factor. The study is still ongoing and it is worth looking forward to updating the long-term survival data.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
Zanubrutinib, a BTK inhibitor, was used in combination with immunochemotherapy in the first-line treatment of DLBCL.
Author notes
Asterisk with author names denotes non-ASH members.